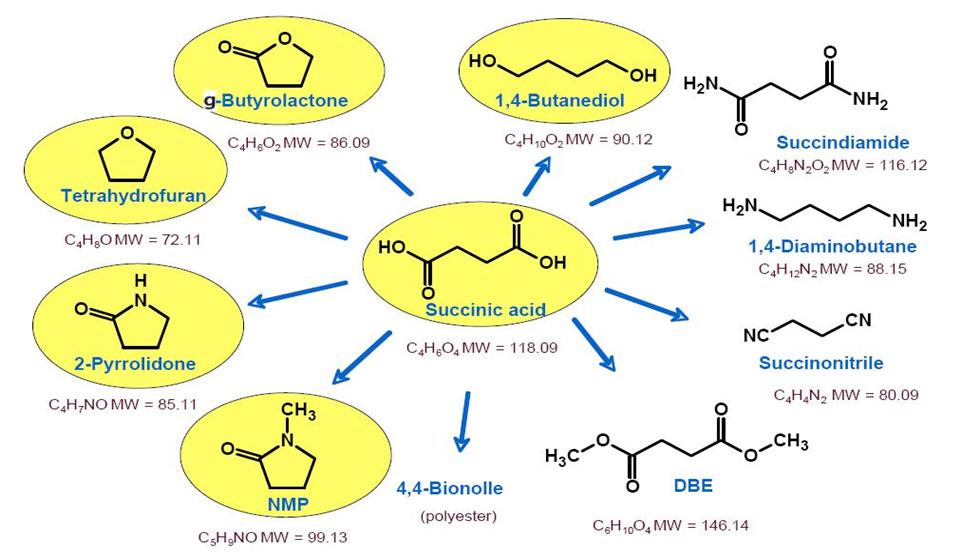
Succinic acid
Genetika Research Center developed advance technology of succinic acid production
|
Succinic acid (IUPAC systematic name: butanedioic acid; historically known as spirit of amber) is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained.
The carboxylate anion is called succinate and esters of succinic acid are called alkyl succinates.
Physical properties
At room temperature, pure succinic acid is a solid that forms colorless, odorless crystals. It has a melting point of 185 °C and a boiling point of 235 °C. It is a diprotic acid.
|
 |
Biochemical role
Succinate is a component of the citric acid cycle and is capable of donating electrons to the electron transfer chain by the reaction:
succinate + FAD → fumarate + FADH2.
This is catalysed by the enzyme succinate dehydrogenase (or complex II of the mitochondrial ETC). The complex is a 4 subunit membrane-bound lipoprotein which couples the oxidation of succinate to the reduction of ubiquinone. Intermediate electron carriers are FAD and three Fe2S2 clusters part of subunit B
|
For more information please send request here